The incidence of mouth and throat cancer is on the rise due to transmission of the human papilloma virus (HPV), but the “silver lining” of this phenomenon is doctors at Memorial Sloan Kettering (MSK) can significantly reduce the intensity of treatment and improve quality of life for these patients.
The most common treatment modalities for oropharynx cancer (OPC) include concurrent chemoradiation therapy, robotic surgery with postoperative radiation or chemoradiation, and induction chemotherapy followed by chemoradiation therapy — the combination of which is known as “trimodality therapy”.
Trimodality therapy can leave patients with debilitating side effects, including long-term issues with speech and swallowing.
At MSK, treatment of oropharynx cancers with trimodality therapy is now less than 8 percent (1) compared with the North American average of 41 percent (2).
This massive decrease is due to a number of factors, but has occurred primarily because MSK employs a multidisciplinary approach to treatment planning as well as careful upfront patient selection for robotic surgery or nonsurgical treatment.
The changing demographic of oropharynx cancer: including HPV as a parameter
Historically, head and neck squamous cell cancer has been described as “an homogenous disease of multiple anatomic sites that is strongly associated with tobacco and alcohol use” (3). But the increase in HPV infection means that this virus is now the major cause of squamous cell carcinomas of the oropharynx, responsible for more than 80 percent of cases.
This increase has been seen predominantly in developed countries, particularly in younger men, and is associated with sexual behavior (4).
Importantly, although HPV-related OPC is a “rapidly emerging disease” (5), HPV-positive status is linked with a more favorable prognosis, as compared with HPV-negative disease (6).
HPV-positive OPC shares histologic features with HPV-negative OPC, but they have fundamental biological differences. For example, HPV-positive OPC exhibits a loss of TRAF3, activating mutations of PIK3CA, amplification, and E2F1 (7). This has important implications for targeted therapy.
Yet this critical, and now widely recognized, parameter has not been accounted for in the current seventh edition of the American Joint Committee on Cancer (AJCC) staging system, which means that many OPC patients are still being treated with more aggressive therapy than is necessary.
To address this, MSK physicians took part in the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S), a global initiative formed to incorporate HPV data in providing prognostic information (3), (5).
An independent validation of the ICON-S system recently demonstrated superior stratification of overall and progression-free survival by this system as compared with the seventh edition of AJCC (3).
In 2014, MSK’s Head and Neck Service implemented a weekly disease management team review — including specialists from surgery, radiation oncology, and medical oncology — of every patient with a diagnosis of squamous cell carcinoma of the oropharynx.
Pathology and radiology results are assessed prior to a case review by a multidisciplinary tumor board. An individualized treatment plan is designed for each patient by team consensus.
Robotic surgery: patient selection is key
Robotic surgery now allows for tumors from the back of the throat to be surgically removed in a minimally invasive fashion with optimal visualization through the open mouth Trans Oral Robotic Surgery (TORS) system.
At MSK, a formal radiology review is performed to identify factors that lead to postoperative chemoradiation including extra capsular spread and high risk for positive margins. Only specifically selected patients that meet these criteria are offered TORS (1).
In non-selective approaches, surgery often leads to positive margins or removing nodes with extensive extracapsular spread of disease requiring postoperative chemoradiation (trimodality) therapy.
Primary surgery may reduce, or possibly even eliminate, the need for postoperative adjuvant chemotherapy or radiation therapy, depending on the final pathology results. Adjuvant therapy de-escalation remains a major rationalization for considering primary surgery over primary chemoradiation therapy.
Another effort to explore the de-intensification of adjuvant therapy following robotic surgery of oropharynx cancer is addressed by a clinical trial (ECOG 3311) that is currently open at MSK.
This trial offers patients, the possibility of being randomized to receive a reduced dose of postoperative radiation or possibly avoid postoperative radiation entirely, based on pathologic parameters after robotic surgery. This national trial will examine feasibility, progression-free survival, and, as secondary endpoints, assess overall survival, swallowing function, and adverse events.
Radiation therapy: review, adaptive replanning, and dose de-escalation
As with surgery, a highly selective approach is taken with regards to patient suitability for radiation therapy (1).
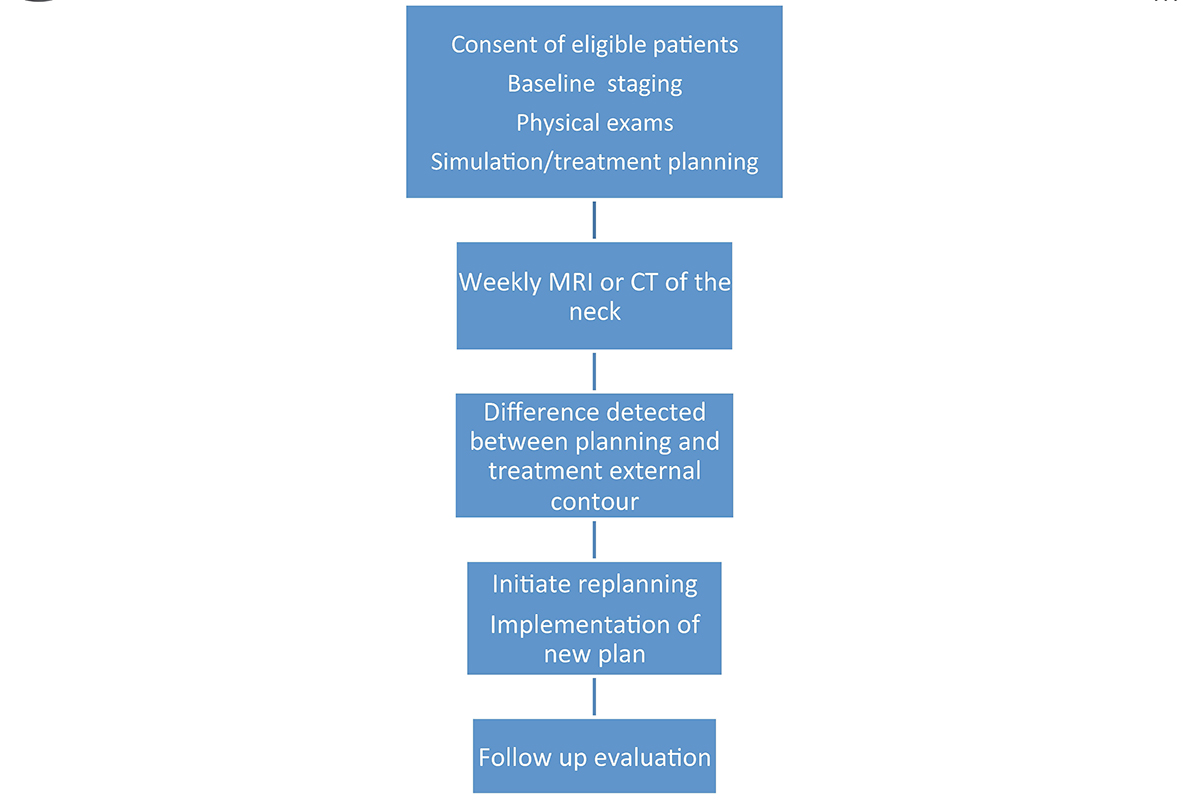
At MSK, all head and neck cancer patients have a “weekly mapping” with MRIs to determine treatment response. This allows the treating physician to adapt the radiation plan in real time to allow for more precise delivery of radiation to the tumor target while sparing the normal tissue.
Historically, all patients received about six to seven weeks of radiation therapy. But because HPV-positive OPC responds better to treatment, our radiation oncologists now de-escalate the radiation dose delivered to normal tissues based on what the MRI shows.
Even if the patient has had a gross total resection, the MRI serves to determine the anatomic changes with respect to all the normal tissues such as parotid glands and submandibular glands. Our radiation oncologists then “replan” the treatment based on each patient’s change, which is why it is called adaptive radiotherapy. Before, we only had one plan and could not account for anatomic changes secondary to weight loss (8).
We offer a variety of carefully selected clinical trials offering promising new approaches for patients who are interested in participating. Some of these current trials are aimed at de-escalating the intensity of treatment for HPV-related OPC, and are aimed at reducing toxicity and side effects related to therapy. Others are aimed at testing novel therapies. Currently, we have a trial that shows promising results of treating select HPV-positive OPC to 30Gy and chemotherapy. This is a reduction of almost 60 percent (from 70Gy to 30Gy) in radiation therapy. The promising preliminary results will be presented at the American Society of Clinical Oncology annual conference in 2017.
Leveraging patient-reported outcomes to improve treatment of oropharynx cancers
In an era in which HPV-related OPC is affecting an increasing number of younger patients with an excellent overall prognosis (6), there is a recognized need to maximize quality of life throughout and after treatment.
As described, multimodality therapy in various sequences results in short- and long-term toxicities that affect swallowing, eating, and communication (9).
Measuring patient-reported outcomes (PROs) represents a vital component in the treatment of OPC at MSK. Validated quality of life instruments, some developed by experts at MSK (10), monitor the wellbeing of patients throughout treatment and beyond. Using a revolutionary new PRO platform, MSK Engage, patients can report their treatment experience to the MSK team. This information will be integrated into their electronic medical record and can then be evaluated by the disease management team.
By measuring PROs as part of clinical care, referrals to ancillary departments — including speech and language pathology, swallowing rehabilitation, social work, and pain management — can be promptly made based on an individual patient’s needs throughout and after treatment.
Understanding PROs also enables the DMT to develop novel treatment paradigms that will maintain high cure rates but minimize side effects in patients with OPC.

