Modeling familial dysautonomia and other NC-related disorders
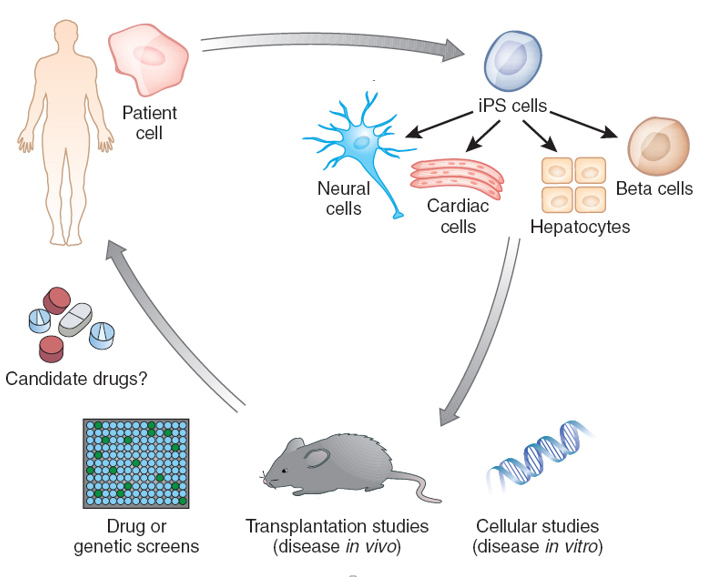
Modeling human disease in a dish.
Familial dysautonomia (FD) is a rare but fatal disorder primarily affecting the peripheral nervous system. We have demonstrated that iPSC technology represents an excellent model of the disease in a dish to study mechanism of disease and to identify and validate promising therapeutic compounds for use in FD patients. Current studies are aimed at understanding variability in disease manifestation among patients and the identification of compounds in our iPSC model to treat later stages of the disease. Other iPSC-based models affecting neural crest lineages include Schwann cell and melanocyte-related disorders, including our effort to model human melanoma in pluripotent stem cells.
Modeling herpes simplex encephalitis and neurodevelopmental disorders
In a multilab collaboration, we have developed methods to model primary herpes simplex encephalitis (HSE) using iPSC cell technology. Those studies have revealed that mutations in a specific pathway (TLR-3 signaling) lead to an autonomous defect of innate immunity in iPSC-derived neurons. The goal of these studies is to further define cellular mechanisms of disease and to model other forms of HSE. Other neurodevelopmental disorders of interest in the lab include autism-spectrum disorders and schizophrenia.
Modeling Parkinson’s disease and other age-related disorders
We have a long-standing interest in modeling Parkinson’s disease (PD) using human iPSC and ESC-derived dopamine neurons.
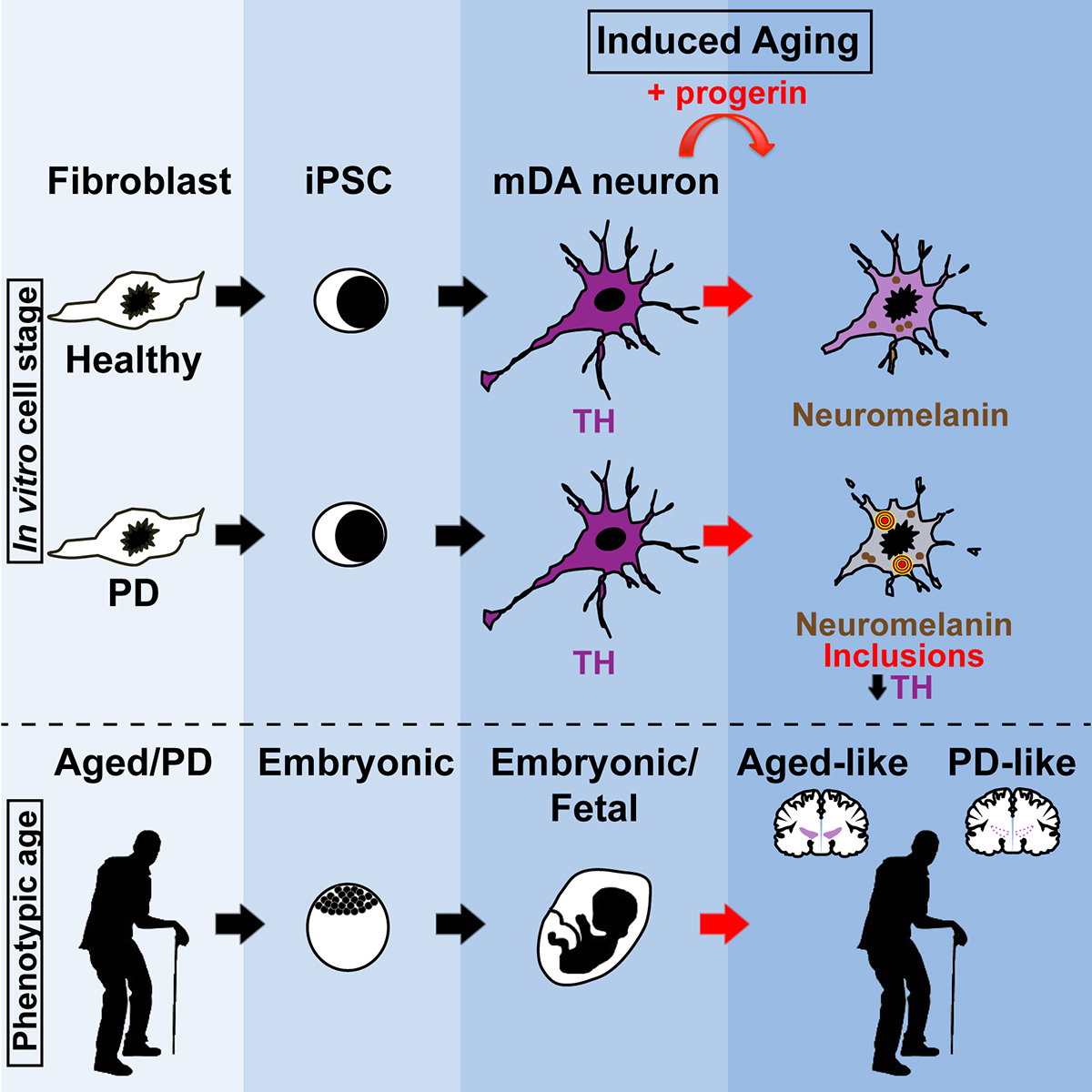
“Induced aging” is a novel strategy to model late-onset features of diseases such as Parkinson’s.