The identification of small-molecule probes and therapeutic lead compounds to address novel biological targets is a significant current challenge in chemical biology and drug discovery. We are engaged in a two-pronged approach to this problem involving diversity-oriented synthesis and rational design as complementary approaches to ligand discovery. Our work in both of these areas is influenced at multiple levels by insights from natural products. At the core of these efforts is the development of new chemical methodologies to provide flexible, efficient, systematic access to these structures. We leverage multidisciplinary collaborations with biologists to evaluate these molecules to probe key biological processes and to explore new therapeutic opportunities in cancer and infectious diseases.
[ Video and Slide Presentation ]
Diversity-Oriented Synthesis and Rational Drug Design - New Chemical Probes for Biology and Medicine
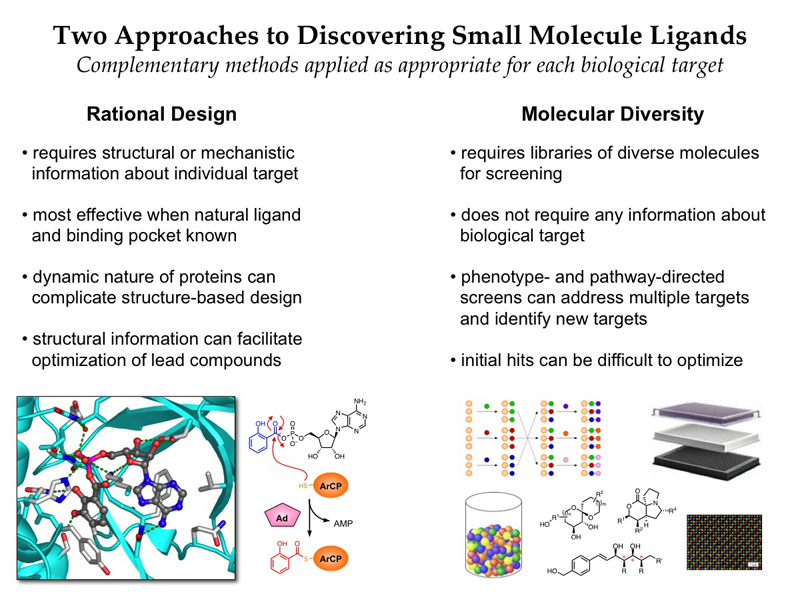
Diversity and Design — Two approaches to identifying new small molecule ligands for chemical biology and drug discovery research
Advances in genomic technologies and molecular cell biology have revealed myriad new biological targets that are implicated in human diseases. However, our ability to translate these discoveries into new therapeutics is severely limited by a major current challenge in chemistry, the identification of small-molecule probes to address these targets. This can be attributed to the fact that existing small-molecule drugs address only a very small subset of ≈200 protein targets encoded in the human genome (≈1%). Efforts to expand beyond this narrow range of biological targets have been thwarted by a heavy focus on a correspondingly narrow range of complementary chemical structures in modern drug discovery. To address this critical problem, our laboratory leverages insights from natural products to develop new small-molecule probes via rational drug design and diversity-oriented synthesis. At the heart of our program lies a deep interest in advancing the frontiers of synthetic organic chemistry and biomedical research.
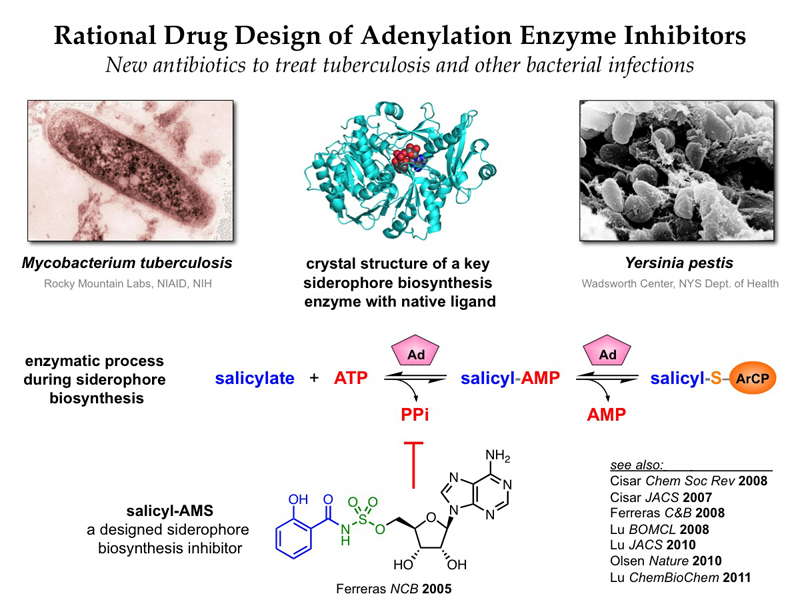
Rational Drug Design — Designed adenylation enzyme inhibitors such as salicyl-AMS are new potential antibiotics
In the area of rational drug design, we are using a natural product-based sulfonyladenosine inhibitor design platform to target a variety of adenylation enzymes. This mechanistic superfamily of enzymes is involved in a wide range of biological processes, and many are promising new antibacterial and anticancer targets. Using this sulfonyladenosine design platform, we have developed inhibitors of several natural-product biosynthesis pathways that are involved in bacterial virulence and pathogenicity. We have identified a number of promising lead compounds and are investigating their potential as new antibiotics to combat pathogenic bacteria such as Mycobacterium tuberculosis, Staphylococcus aureus, and Pseudomonas aeruginosa. Notably, many pharmaceutical companies have abandoned their efforts in antibiotic drug discovery in recent years while antibiotic-resistance is growing rapidly in the clinic, emphasizing the need for continuing advances by academic researchers to combat this growing threat. Recently, we have also applied similar sulfonyladenosine design strategies to develop protein-based probes of eukaryotic E1 activating enzymes that catalyze key steps in ubiquitination and related processes, revealing profound active site remodeling during catalysis.

Diversity-Oriented Synthesis — Novel natural product-based libraries target under-represented regions of chemical space
In the area of diversity-oriented synthesis, we are developing discovery libraries based on privileged structural motifs from natural products. Such structures have a demonstrated ability to bind diverse biological targets, including challenging targets that are intractable to conventional synthetic drug-like molecules. Moreover, they have distinct structural features compared to existing synthetic drugs, and these same features have been correlated with improved clinical success. Thus, these natural product-based libraries are designed to access underexploited regions of chemical structure space to address a complementary spectrum of biological targets compared to conventional synthetic drugs. Importantly, many of the existing approaches to synthesizing these structures are unsuitable for diversity-oriented synthesis, due to the exceptional requirements for reaction efficiency and flexibility. Thus, we are presented with numerous opportunities to develop new chemical methodologies with broader applications in organic synthesis. Our current synthetic targets include spiroketals, polycyclic alkaloids, macrocycles and medium rings, and oxygen heterocycles.
We leverage multidisciplinary collaborations with biologists to evaluate the molecules we synthesize, with particular interests in cancer, infectious diseases, and metabolic disorders. This collaborative approach brings together the strengths of both chemists and biologists and is a critical aspect of our program. Our Tri-Institutional Research Program, which encompasses Sloan-Kettering, Cornell University, and the Rockefeller University, provides a fertile environment for these efforts. The small molecule probes we are discovering are powerful tools for studying fundamental questions in biology and for validating new therapeutic targets in model systems. These molecules also provide valuable starting points for developing new, mechanism-based therapeutics.
For more details, see our Research Projects page.

Chemistry and Chemical Biology Research at MSKCC -- The new MSKCC Zuckerman Research Center opened in summer 2006 and the Tan Lab moved into the top (21st) floor on September 27! (top) Building construction from July 20, 2004 thru June 20, 2005. (bottom) Current view to the southeast from the 21st floor.