The immune system responds to viral infections by introducing a complex interplay of immune cells and factors, whose goal is to eliminate the intruding pathogen. One such effector cell in the antiviral response is the Natural Killer (NK) cell, which secretes inflammatory cytokines and directly kills infected cells. The focus of our research is aimed at defining the signals that NK cells require to mount an effective immune response against pathogens. We are also exploring the regulatory mechanisms keeping NK cells in check, thereby safeguarding against potential autoimmunity.
Understanding the biology of NK cells and how these cells of the immune system specifically attack virally infected and cancerous cells, but not healthy cells, we will be able to develop more effective preventative and therapeutic approaches in the battle against infectious diseases and cancer.
Current Projects
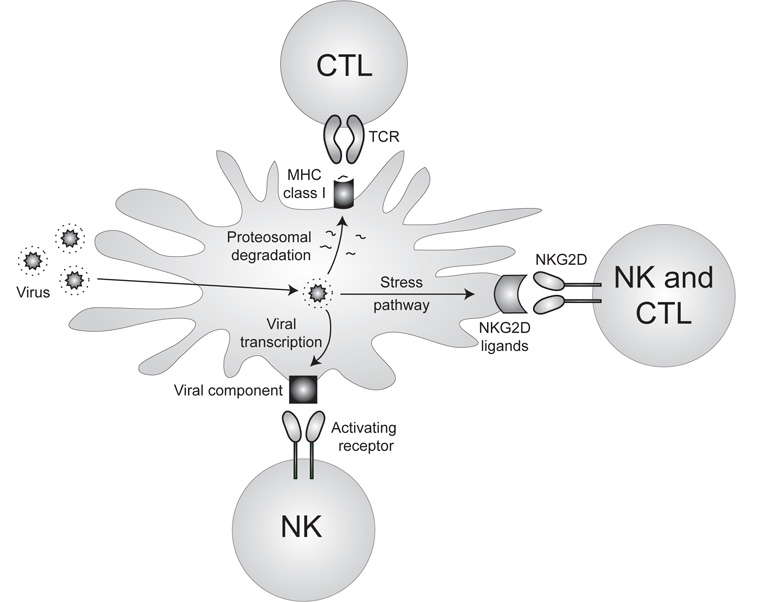
Figure 1 — Specific recognition of virally infected cells by the immune system.
Although traditionally classified as cells of the innate system, NK cells closely resemble T cells in phenotype and function. More recently NK cells have been thought to represent an evolutionary link between the non-specific cells of innate immunity and the antigen-specific B and T cells of adaptive immunity. If NK cells are indeed a “bridge” between innate and adaptive immunity, they might possess certain attributes of both. We now have evidence that several hallmark characteristics of the adaptive immune system (including developmental selection, clonal expansion, and immune memory) can be found in NK cells. We are interested in further understanding the molecular and cellular mechanisms involved in NK cell development, effector function, and generation of memory.
We have recently demonstrated that similar to antigen-specific T cells, a subset of NK cells bearing the activating Ly49H receptor, which specifically binds the viral glycoprotein m157, can proliferate 100-1000 fold in lymphoid and non-lymphoid organs following mouse cytomegalovirus infection. The activated NK cells then became long-lived and self-renewing months after infection. These “memory” NK cells were able to rapidly mount effector functions and undergo secondary expansion to provide protective immunity. The implications of our findings can potentially have profound influences in how individuals are vaccinated. However, the signals that drive NK cell activation, expansion, and memory remain unclear.
Thus, we are interested in the following questions:
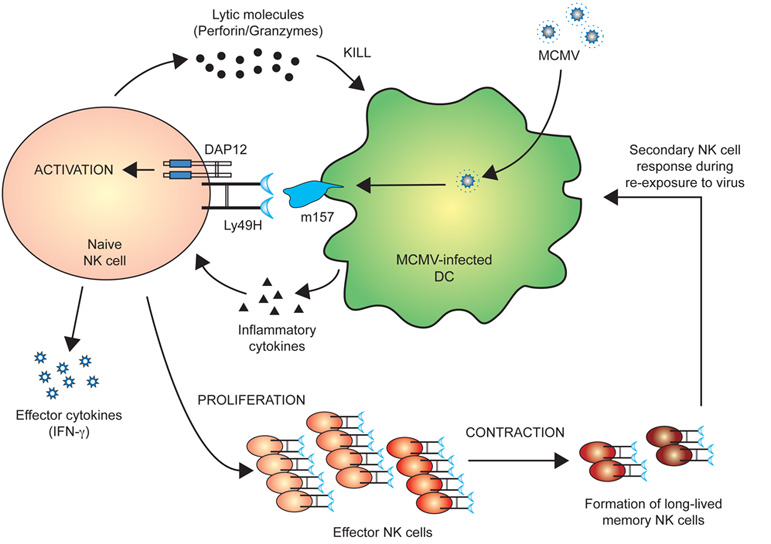
Figure 2 — Specific NK cell response against MCMV.
- What are the signals NK cells receive during development, and how do these cellular and molecular interactions influence the function of mature NK cells?
- What are the molecular requirements that drive NK cell differentiation and effector function?
- How do the early cytokine signals NK cells receive promote their proliferation and memory cell formation during viral infection?
- What are the regulatory mechanisms that keep NK cells in check once they become activated?
- What are the late signals and interactions responsible for the maintenance of long-lived NK cells?
- Can we engineer recombinant pathogens expressing specific antigens in order to vaccinate via the NK cell compartment?