One of the main topics of our laboratory is to investigate novel therapeutic strategies to mitigate graft-versus-host disease (GVHD) in transplanted recipients using murine HSCT models. The following are summaries of projects that address the mechanisms underlying the pathophysiology and amelioration of GVHD:
Autophagy gene Atg16l1 protects against lethal T cell alloreactivity mediated by dendritic cells
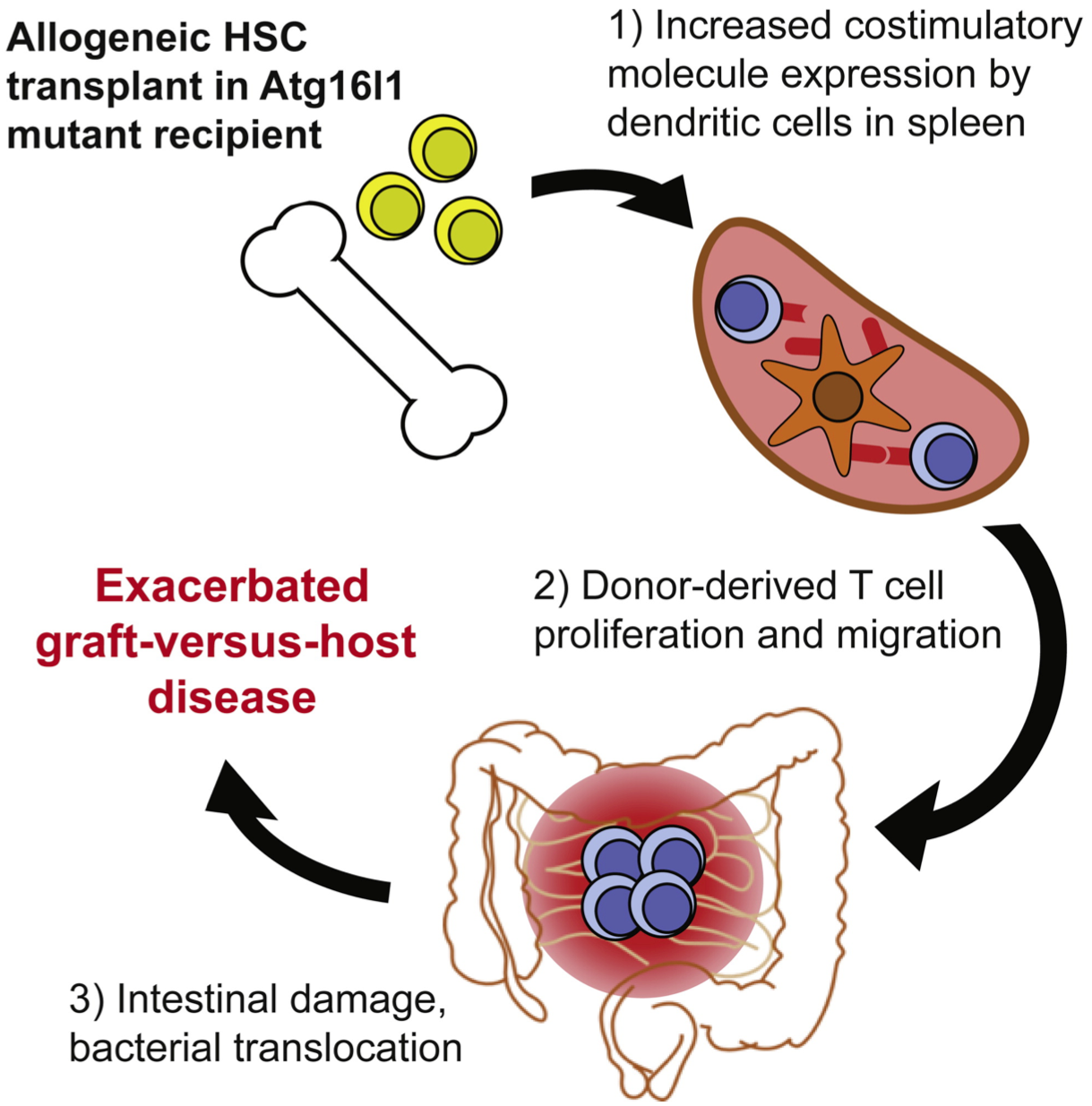
Vanessa M. Hubbard-Lucey, Yusuke Shono et al. Immunity, Volume 41, Issue 4, p579–591, 16 October 2014
Atg16L1 mediates the cellular degradative process of autophagy and is considered a critical regulator of inflammation based on its genetic association with inflammatory bowel disease. In the paper recently published by Yusuke Shono et al. we demonstrated that Atg16L1 deficiency leads to an exacerbated graft-versus-host disease (GVHD) in a mouse model of allo-HSCT. Atg16L1-deficient allo-HSCT recipients with GVHD displayed enhanced T cell proliferation due to increased dendritic cell (DC) numbers and expression of costimulatory moleculs. Reduced autophagy within DCs was associated with lysosomal abnormalities and decreased amounts of A20, a negative regulator of DC activation. These data suggested a role of Atg16L1 and autophagy in limiting a DC-mediated response during inflammatory disease, such as GVHD.
Pathophysiology of Thymic GVHD
Along with conditioning related damage we and others have previously demonstrated that GVHD can cause further damage to the thymus in allo-HSCT. Thymic GVHD results in loss of double positive thymocytes, thymic epithelial cells, and architectural integrity. One of the interests of our lab is to explore the pathophysiology of thymic GVHD and to develop strategies that can ameliorate its effects, thereby enhancing T cell reconstitution in recipients of allo-HSCT.
Brain GVHD
Although skin, gut, liver, thymus, and lung are all GVHD targets, neurological complications have also been reported following allo-HSCT. We have recently demonstrated that the central nervous system (CNS) can be a direct target of alloreactive T cells following allo-HSCT in mice. We found significant infiltration of the CNS with donor T lymphocytes and cell death of neurons and neuroglia in allo-HSCT recipients with GVHD. Importantly, we also found that allo-HSCT recipients with GVHD had deficits in spatial learning/memory and demonstrated increased anxious behavior. These findings highlight CNS sensitivity to damage caused by alloreactive donor T cells, and represent the first characterization of target cell subsets as well as neurological complications during GVHD. Therefore, these clinically relevant studies offer a novel and rational explanation for the well-described neurological symptoms observed after allo-HSCT.

